The HCBI @ Allston was designed to allow researchers in the SEAS facility access to the latest and best imaging technologies. The facility was designed to be an “Evergreen” microscopy facility. Due to our leasing structure, imaging systems will be evaluated every 2-3 years. This ensures that the facility remains relevant and is never in possession of outdated equipment.
Resource Quick Reference
The table below serves as a quick reference guide for equipment available in the core. For equipment specifics, keep scrolling or as we prefer, key word search.
| Microscopes | Graphics Workstation** | |
| LSM 900 Bio | Sensitive widefield fluorescence imaging. Can perform standard multicolor confocal imaging, spectral imaging and create patterned excitation for FRAP/optogenetics experiments. | Graphics Workstation 1: Deconvolution / Airyscan Processing |
| LSM 900 Materials | DIC, phase contrast, polarization, reflected light and fluorecence. Image in fluorescence or reflected light mode. | Graphics Workstation 2: Intellisis ML Segmentation |
| Axio Scan | 100-slide capacity to perform poloarized light imaging and image samples stained with both colormetric, and fluorescent dyes. | ** all workstations perform standard ZEN Blue functions (stitching, image export) |
You Are Welcome to Join Us

Training and Onboarding
The facility is a paid use imaging core. Users are trained by dedicated imaging scientists and assisted in planning and conducting their imaging experiments as needed. Users of the facility can be internal university users or external clients. All training and consultation time is free, but usage no the microscopes is charged.
Your must register to initiate the onboarding and training process.
Equipment fees can be found here.

Learn more about the HCBI
To learn more about the HCBI and it’s mission visit the main HCBI website.

Meet the team
The facility is staffed once a week (Wednesday) by staff.
Alex Lovely is the staff member in charge of the OIC facility. Alex got his PhD from the biology department at Northeastern University in March 2021. His research was focused on the use of multiplexed in situ hybridization techniques (HCR FISH) in the investigation of gene expression during limb regeneration in axolotl salamanders. Alex is based in Biological Laboratories 16 Divinity Ave., Room 2058B Cambridge, MA, 02138.
Email: alexander_lovely@fas.harvard.edu
This Year’s Equipment Summary
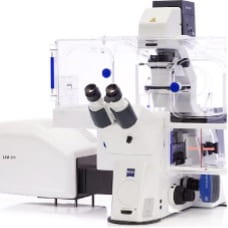
LSM 900 Bio confocal
This LSM 900 comes fully loaded for all of your bioengineering needs. A Hamamatsu Flash 4.0 camera allows for fast, sensitive widefield fluorescence imaging. The confocal scan head can perform standard multicolor confocal imaging, spectral imaging and create patternned excitation for FRAP/optogenetics experiments. Additionally, an Airyscan 2.0 detector is availible to improve resolution beyond the difration barrier and increase confocal sensitivity. The entire system is fully motorized allowing for autofocusing over large fields of view. Incubation and environmental control will keep your samples happy for the duration of the experiment.
Objectives: 2.5x/0.085, 10x/0.45 air, 20x/0.8 air, 40x/1.2 water, 40x/1.3 oil
Laser lines: 405, 488, 561, 640
Commonly imaged dyes: DAPI, Alexa 488/GFP, Alexa 555/568/tdTomato, Alexa 647

LSM 900 material confocal
This LSM 900 is configured to be a materials workhorse. It comes equiped with black and white and color cameras that can image a wide range of widefield contrasts: DIC, phase contrast, polarization, reflected light and fluorecence. The confocal scan head can image in fluorescence or relected light mode.
Objectives: 2.5x/0.085 air, 5x/0.3 air, 10x/0.25 air, 20x/0.4 air (phase ring) 20x/0.5 air, 40x/1.0 water dipping, 50x/0.55 air,
Laser lines: 405, 488, 561, 640
Commonly imaged dyes: DAPI, Alexa 488/GFP, Alexa 555/568/tdTomato, Alexa 647

Axio Scan.Z7
Have you ever looked at a giant pile of slides, and wondered how you’ll find the time to image them all? The Axio Scan.Z1 is your answer! This high speed slide scanner has an astonishing 100-slide capacity, and can image samples stained with both colormetric and fluorescent dyes. This particular system is configured with the ability to perform polarized light imaging as well.
LED excitation: 385, 430, 475, 511, 555, 590, 630 nm
Objectives: 5x/0.16 air, 10x/0.45 air, 20x/0.8 air
Filter sets: quadruple bandpass (Blue, Green, Red, Far-Red), DAPI/BFP, 488/GFP, 555/568/tdTomato, 594/mCherry, 647/Cy5
Best dye combinations: DAPI/488/568/647Best dye combinations: DAPI/488/568/647; CFP/YFP/mCherry